BIOSENSOR & BIOCHIP SURFACES
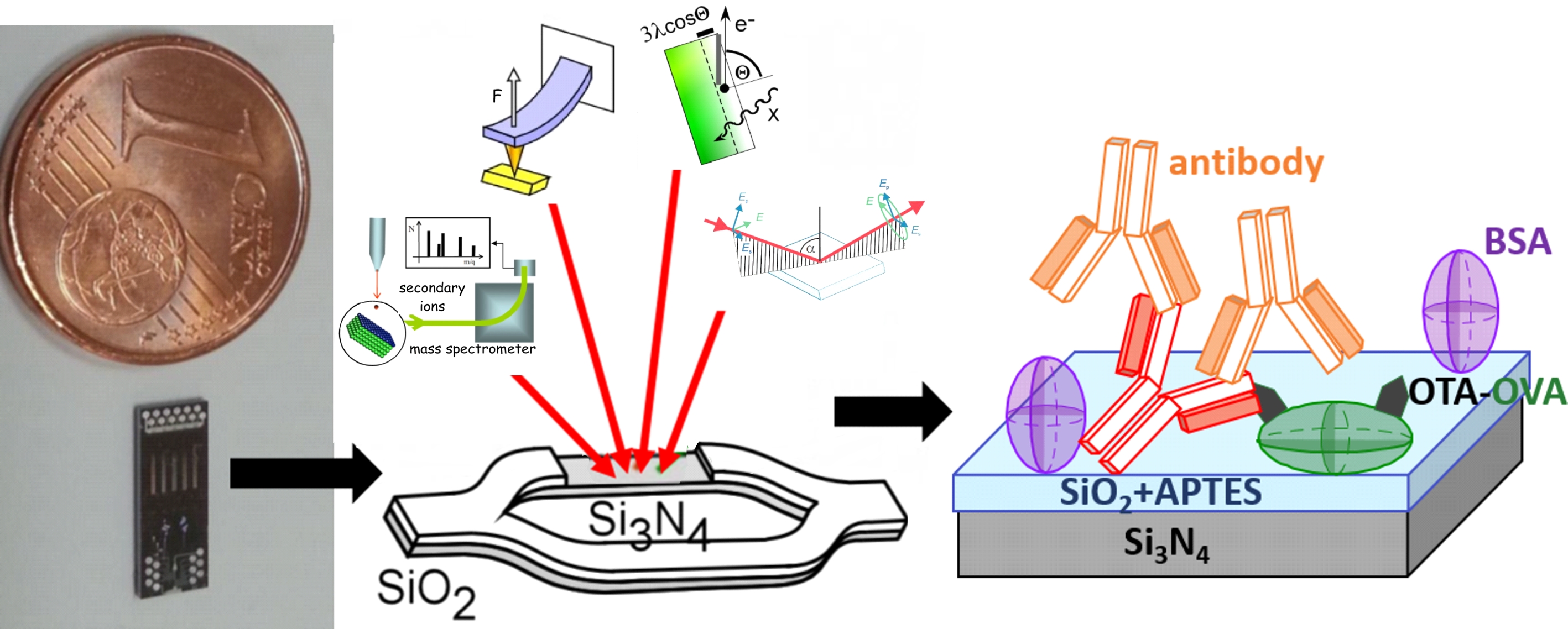
BIOSENSOR SURFACES
Biosensors transduce information about amount of chemical substances, analytes, into signals that can be recorded. We participate in multidisciplinary projects targeting various analytes, such as cancer markers and gene mutations, relevant for early diagnosis of human diseases (PYTHIA EU project) or allergens, mycotoxins and pesticides, relevant for food safety (FOODSNIFFER EU project). Our research is focused on biosensor surfaces, where molecular recognition of analytes takes place, a process that lies at the heart of biosensing function. We apply complementary surface science techniques (e.g. AFM, TOF-SIMS, ellipsometry, XPS) supplemented with biochemical assays. Over past decade we have developed various appropriate surface analysis methodologies:
i) Optimizing bioreceptor immobilization at biosensor surface using physical adsorption1, covalent bonding2, specific binding3;

Fig.1. Analyte binding efficiency of bioreceptor (immunoglobulin G antibody) covalently immobilized on silicon substrate following two functionalization methods.
ii) Determining surface amounts of all different molecules applied in multi-step and multi-component protocols4;
iii) Evaluating biding stoichiometry more accurately than from biosensor response5;
iv) Determining directly orientation of bioreceptor (e.g. antibody2,4,6 or, streptavidin7) in multi-component assays.
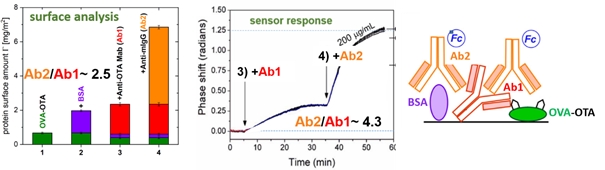
Fig.2. Composition analysis of biomolecular layers on the sensor surface. Application of the surface analysis (TOF-SIMS and ellipsometry) enables evaluation of molecules binding ratio, more accurate as compared with sensor response analysis, as well as molecules orientation examination.
BIOCHIP SURFACES
Since classical analytical technologies are expensive and time consuming, as bound to laboratories, high hopes are placed on cost-effective microarrays of miniaturized biosensors. Such label-free opto-electronic biochips (e.g. PHYTIA or FOODSNIFFER) enable rapid and synchronous multi-analyte detection with a single chip. Therefore, precise spatially resolved immobilization of bioreceptors on respective miniaturized biosensors is highly demanded. For this purpose, we analyse biochip surfaces following bioprinting with a robotic microarray spotter. This has resulted in:
v) Characterization of fast and effective bioprinting of nanolitre droplets of bioreceptors resulting in surface amount twice higher than those of classically pipetted microlitre drops (hand macro-spotting)8
vi) Comparison of different protocols to form DNA arrays based on biotin – streptavidin system, including imaging of bioreceptor distribution within single microarray spots, resulting in the most effective procedure for gene mutation detection3
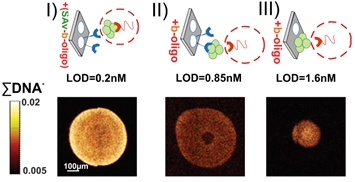
Fig.3. Chemical images of DNA spots bioprinted using different immobilization protocols applying biotin-streptavidin system.
vii) Conditions, established for uniform bioreceptor immobilization within arbitrary patterns, that boost considerably (5-fold) assay detection sensitivity9
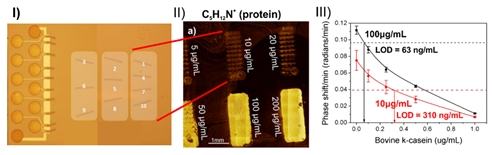
Fig.4. Label-free analysis of bioprinted patterns (II) optimizes bioreceptors immobilization on respective on-chip biosensors (I) resulting in 5-fold reduction of limits of detection (III).
viii) Optimized bioprinting onto photolithographic micropatterns, that results in selective immobilization with sub-micrometer spatial resolution10, suitable for the first all-silicon spectroscopic lab-on-chip.
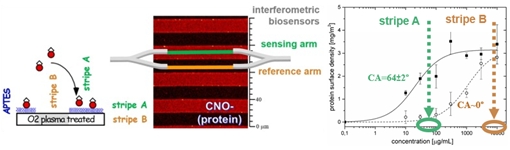
Fig.5. Evaluation of the spatially selective bioreceptor immobilization method, producing alternating stripes that match two distinct arms of on-chip interferometric biosensors.
Our research is performed in the frame of different projects:
Silicon biosensing surfaces functionalization with many molecules: direct analysis of antibody orientation and antigen binding efficiency with secondary ion mass spectroscopy (NCN UMO-2016/21/N/ST5/00880)
Foodsniffer: FOOD Safety at the point-of-Need via monolithic spectroscopic chip identIFying harmFul substances in frEsh pRoduce (FP7-ICT8-318319 & 3132/7.PR/2014/2)
Protein immobilisation to film surfaces and micro-patterns of conjugated polymer in the aspect of biosensors applications (NCN UMO-2011/03/N/ST5/04764)
PYTHIA: Monolithically integrated interferometric biochiPs for label-free earlY deTection of Human dIseAses (FP7-ICT2-224030 & 1460/7. PR UE/2010/7)
References:
1 "Protein adsorption and covalent bonding to silicon nitride surfaces modified with organo-silanes: comparison using AFM, angle-resolved XPS and multivariate ToF-SIMS analysis", K. Awsiuk, A. Budkowski, A. Psarouli, P. Petrou, A. Bernasik, S. Kakabakos, J. Rysz, I. Raptis, Colloids and Surfaces B: Biointerfaces 110 (2013) 217-224
2 "Surface density dependent orientation and immunological recognition of antibody on silicon: TOF-SIMS and surface analysis of two covalent immobilization methods" K. Gajos, K. Szafraniec, P. Petrou, A. Budkowski, Applied Surface Science 518 (2020), 146269
3 "Imaging and spectroscopic comparison of multi-step methods to form DNA arrays based on the biotin-streptavidin system" K. Gajos, P. Petrou, A. Budkowski, K. Awsiuk, A. Bernasik, K. Misiakos, J. Rysz, I. Raptis, S. Kakabakos, Analyst 140 (2015), 1127–1139
4 "Indirect immunoassay on functionalized silicon surface: Molecular arrangement, composition and orientation examined step-by-step with multi-technique and multivariate analysis” K. Gajos, A. Budkowski, V. Pagkali, P. Petrou, M. Biernat, K. Awsiuk, J. Rysz, A. Bernasik, K. Misiakos, I. Raptis, S. Kakabakos, Colloids and Surfaces B: Biointerfaces 150 (2017), 437-444
5 "Protein adsorption/desorption and antibody binding stoichiometry on silicon interferometric biosensors examined with TOF-SIMS" K. Gajos, A. Budkowski, P. Petrou, V. Pagkali, K. Awsiuk, J. Rysz, A. Bernasik, K. Misiakos, I. Raptis, S. Kakabakos, Applied Surface Science 444 (2018), 187-196
6 "Orientation and biorecognition of immunoglobulin adsorbed on spin-cast poly(3-alkylthiophenes): impact of polymer film crystallinity" K. Awsiuk, A. Budkowski, P. Petrou, M. M. Marzec, M. Biernat, T. Jaworska-Gołąb, J. Rysz, Colloids and Surfaces B: Biointerfaces 148 (2016), 278-286
7 "Orientation of Biotin-Binding Sites in Streptavidin Adsorbed onto the Surface of Polythiophene Films" K. Awsiuk, P. Petrou, A. Thanassoulas, J. Raczkowska, Langmuir 35 (2019), 3058-3066
8 "Contact pin-printing of albumin-fungicide conjugate for silicon nitride-based sensors biofunctionalization: Multi-technique surface analysis for optimum immunoassay performance" K. Gajos, A. Budkowski, Z. Tsialla, P. Petrou, K. Awsiuk, P. Dąbczyński, A. Bernasik, J. Rysz, K. Misiakos, I. Raptis, S. Kakabakos, Applied Surface Science 410 (2017), 79-86
9 "Imaging and chemical surface analysis of biomolecular functionalization of monolithically integrated on silicon Mach-Zehnder interferometric immunosensors" K. Gajos, M. Angelopoulou, P. Petrou, K. Awsiuk, S. Kakabakos, W. Haasnoot, A. Bernasik, J. Rysz, M. M. Marzec, K. Misiakos, I. Raptis, A. Budkowski, Applied Surface Science 385 (2016), 529-542
10 "Spatially selective biomolecules immobilization on silicon nitride waveguides through contact printing onto plasma treated photolithographic micropattern: Step-by-step analysis with TOF-SIMS chemical imaging" K. Gajos, A. Budkowski, P. Petrou, K. Awsiuk, K. Misiakos, I. Raptis, S. Kakabakos, Applied Surface Science 506 (2020), 145002
Contact:
Osoba publikująca: Kamil Awsiuk